SonoVue®
SonoVue® is a second-generation ultrasound contrast agent, designed and optimized for pressure resistance: this is the result of many years of research and development within Bracco.
The research led to the selection of SF6, a gas with low solubility in blood for the gas phase of the microbubbles and a phospholipid monolayer for shell1,2,3. SonoVue® was approved in the European Union by a centralized procedure on 26 March 2001, and is currently marketed in 36 countries worldwide. Today, the possible clinical applications of CEUS are listed in the guidelines published by the Federation of European Ultrasound Societies (EFSUMB)4.
Physical and chemical characteristics
| ready-to-use suspension / vial | 5 ml |
| number of microbubbles / ml suspension | approx. 2 · 108 |
| gas content (SF 6) / ml suspension | 8 μl |
| average diameter of microbubbles | approx. 2,5 μm |
| microbubble size range | 1 - 11 μm (90% < 6 μm, 99% <11 μm) |
| osmolality | approx. 290 mOsmol / Kg |
| viscosity | < 2 mPa · s |
| pH-value | 4,5 - 7,5 |
| stability after preparation | 6 hours |
Presentation
SonoVue® is presented as a kit. The needleless kit includes all the necessary material for
SonoVue® preparation:
- 5 ml saline 0.9 % pre-filled syringe
- a minispike
- a vial containing gas and lyophilized powder.
1) Design of an ultrasound contrast agent for myocardial perfusion.
Schneider M.Echocardiography. 2000 Aug;17(6 Pt 2):S11-6. Review.
2) BR1: a new ultrasonographic contrast agent based on sulfur hexafluoride-filled microbubbles.
Schneider M., Arditi M., Barrau MB., Brochot J., Broillet A., Ventrone R., Yan F.Invest Radiol. 1995 Aug;30(8):451-7.
3) Characteristics of SonoVue®.
Schneider M.Echocardiography. 1999 Oct;16(7, Pt 2):743-746.
4) EFSUMB CEUS guidelines
5) SonoVue® SPC
6) Contrast enhanced ultrasound for the characterization of focal liver lesions – Diagnostic accuracy in clinical practice (DEGUM multicenter trial)
Strobel D., Seitz K., Blank W., Schuler A., Dietrich C., von Herbay A., Friedrich-Rust M., Kunze G., Becker D., Will U., Kratzer W., Albert FW., Pachmann C., Dirks K., Strunk H., Greis C., Bernatik T.Ultraschall Med. 2008 Oct;29(5):499-505. doi: 10.1055/s-2008-1027806.
7) Contrast-Enhanced Ultrasound (CEUS) for the characterization of focal liver lesions - prospective comparison in clinical practice: CEUS vs. CT (DEGUM multicenter trial). Parts of this manuscript were presented at the Ultrasound Dreiländertreffen 2008, D
Seitz K., Strobel D., Bernatik T., Blank W., Friedrich-Rust M., Herbay Av., Dietrich CF., Strunk H., Kratzer W., Schuler A.Ultraschall Med. 2009 Aug;30(4):383-9. doi: 10.1055/s-0028-1109673. Epub 2009 Aug 17.
8) Contrast-enhanced ultrasound (CEUS) for the characterization of focal liver lesions in clinical practice (DEGUM Multicenter Trial): CEUS vs. MRI--a prospective comparison in 269 patients.
Seitz K., Bernatik T., Strobel D., Blank W., Friedrich-Rust M., Strunk H., Greis C., Kratzer W., Schuler A.Ultraschall Med. 2010 Oct;31(5):492-9. doi: 10.1055/s-0029-1245591. Epub 2010 Jul 22.
9) Prospective Multicenter Trial Evaluating a Novel Method of Characterizing Focal Liver Lesions Using Contrast-Enhanced Sonography.
Leen E., Ceccotti P., Kalogeropoulou C., Angerson WJ., Moug SJ., Horgan PG.AJR Am J Roentgenol. 2006 Jun;186(6):1551-9.
10) Real-time contrast-enhanced ultrasound in the evaluation of focal liver lesions: diagnostic efficacy and economical issues from a French multicentric study.
Tranquart F., Correas JM., Ladam Marcus V., Manzoni P., Vilgrain V., Aube C., Elmaleh A., Chami L., Claudon M., Cuilleron M., Diris B., Garibaldi F., Lucidarme O., Marion D., Beziat C., Rode A., Tasu JP., Trillaud H., Bleuzen A., Le Gouge A., Giraudeau B., Rusch E.J Radiol. 2009 Jan;90(1 Pt 2):109-22. French.
11) Assessment of systolic left ventricular function: a multi-centre comparison of cineventriculography, cardiac magnetic resonance imaging, unenhanced and contrast-enhanced echocardiography.
Hoffmann R., von Bardeleben S., ten Cate F., Borges AC., Kasprzak J., Firschke C., Lafitte S., Al-Saadi N., Kuntz-Hehner S., Engelhardt M., Becher H., Vanoverschelde JL.Eur Heart J. 2005 Mar;26(6):607-16. Epub 2004 Dec 17.
12) The clinical applications of contrast echocardiography.
Olszewski R., Timperley J., Szmigielski C., Monaghan M., Nihoyannopoulos P., Senior R., Becher H.Eur J Echocardiogr. 2007 Jun;8(3):S13-23. Review. Erratum in: Eur J Echocardiogr. 2007 Oct;8(5):308. Cezary, Szmigielski [corrected to Szmigielski, Cezary]; Nihoyannopoulis, Petros [corrected to Nihoyannopoulos, Petros].
Important Safety Information on SonoVue® (8 microlitres/ml powder and solvent for dispersion for injection) Use in Critically ill Patients
SonoVue® (8 microlitres/ml powder and solvent for dispersion for injection) is an ultrasound contrast agent consisting of a suspension of tiny microbubbles (most microbubbles between 2 and 9 microns) with a unique structure made of an inert gas, sulphur hexafluoride, and a phospholipid shell that provides stability and prevents microbubble coalescence. When injected intravenously, SonoVue® strongly enhances the echogenicity of blood, which results in an improved signal-to-noise ratio.
Important New Safety Information
Following an evaluation of the benefits and risks of SonoVue® use in critically ill patients made to by the Committee on Human Medicinal Products (CHMP) of the European Medicines Agency (EMA), the decision was made to:
- delete the contraindications for SonoVue® use in patients with recent acute coronary syndrome or clinically unstable ischaemic cardiac disease, including: evolving or ongoing myocardial infarction, typical angina at rest within last 7 days, significant worsening of cardiac symptoms within last 7 days, recent coronary artery intervention or other factors suggesting clinical instability (for example, recent deterioration of ECG, laboratory or clinical findings), acute cardiac failure, Class III/IV cardiac failure, or severe rhythm disorders;
- to insert SonoVue® use information on these patient populations into the Special Warnings and Precautions for Use section of the Product Information.
Since its launch in 2001, four (4) cases of severe cardiac arrhythmia occurred following concomitant administration of SonoVue® and the direct-acting, inotropic agent dobutamine. Of note, these 4 cases occurred in patients with either ongoing myocardial infarction, or other factors suggesting clinical instability, acute cardiac failure, or severe rhythm disorders, where the use of dobutamine is specifically contraindicated. One case, occurred in a patient with dilated cardiomyopathy and severe heart failure. This patient experienced ventricular fibrillation and cardiac arrest during the stress-echo procedure; the adverse events were considered related to the administration of dobutamine by the reporter. In the further clinical course, the patient had multiple cardiac arrests and developed subsequent cardiac and multi-organ-failure. She died 19 days after the reported events.
In view of these rare events and the frequent use of dobutamine for stress echocardiographic procedures, the CHMP also decided to make sure SonoVue® is not used in combination with dobutamine in those patients for which the use of this pharmacologic stressor is contraindicated.
Therefore, the CHMP decided to add the following contraindication to the Product Information of SonoVue®:
"SonoVue® should not be used in combination with dobutamine in patients with conditions suggesting cardiovascular instability where dobutamine is contraindicated".
In summary:
- SonoVue® is no longer contraindicated in patients with recent acute coronary syndrome or clinically unstable ischaemic cardiac disease.
- Prescribers may use SonoVue® in this patient population using extreme caution, after a careful risk/benefit assessment and with close monitoring of vital signs during and after administration.
- SonoVue® should not be used in combination with dobutamine in patients with conditions suggesting cardiovascular instability where dobutamine is contraindicated.
To provide SonoVue® users and prescribers with this new important safety information, Bracco has agreed upon with EMA and sent out a "Dear Healthcare Professional Communication" (DHPC). This letter is intended to minimise any potential risk for the use of SonoVue® in critically ill patients, which may particularly benefit from more accurate diagnostic information obtained after administration of SonoVue®.
The original DHCP letter is attached.
Further Information
SonoVue® is approved for use in:
- Echocardiography
SonoVue® is a transpulmonary echocardiographic contrast agent for use in patients with suspected or established cardiovascular disease to provide opacification of cardiac chambers and enhance left ventricular endocardial border delineation.
- Doppler of macrovasculature
SonoVue® increases the accuracy in detection or exclusion of abnormalities in cerebral arteries and extracranial carotid or peripheral arteries by improving the Doppler signal to noise ratio.
SonoVue® increases the quality of the Doppler flow image and the duration of clinically useful signal enhancement in portal vein assessment.
- Doppler of microvasculature
SonoVue® improves display of the vascularity of liver and breast lesions during Doppler sonography, leading to more specific lesion characterisation.
Call for reporting
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via their national reporting system.
Company Contact Points
Suspected adverse reactions should also be reported to Bracco International BV: [email protected]
Fax: +39-02-21772766
Phone: +39-02-21772327
For further inquiries concerning the information contained in this communication please contact Professional Services:
Please download full Prescribing Information in the "Resources" menu point above.
How to use it
Preparation of SonoVue®
SonoVue® is supplied in a kit containing a vial with phospholipids (lyophilised) in a sulphur hexafluoride atmosphere, a syringe pre-filled with physiological saline (0.9% saline solution) and a Mini-Spike transfer system. The suspension must be reconstituted before application. Reconstituting the microbubbles is not a difficult procedure but it is important to follow the instructions carefully in order to achieve the optimal effect of the contrast medium.

1. Connect the plunger by screwing it clockwise into the syringe.

2. Open the MiniSpike transfer system from the blister pack and remove the syringe cap.
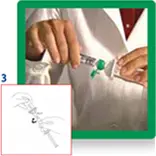
3. Open the transfer system cap and connect the syringe to the transfer system by screwing it in clockwise.
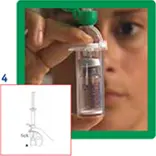
4. Remove the protective disc from the vial. Insert the vial into the transparent sleeve of the transfer system and press firmly to lock the vial in place.

5. Empty the contents of the syringe into the vial by pushing on the plunger.

6. Shake vigorously for 20 seconds to mix all the contents of the vial into a homogeneous milky liquid.

7. Reverse the system and carefully aspirate SonoVue into the syringe.

8. Unscrew the syringe from the transfer system.
Important: do not use if the liquid obtained is not clear and/or if solid parts of the lyophilizate are visible in the suspension.
SonoVue Video
- Preparation and Reconstitution
Indications
SonoVue® is used in ultrasound imaging to increase the echogenicity of blood, which in turn leads to an improvement in the signal-to-noise ratio5.
Echocardiography
SonoVue® is an echocardiographic contrast agent that passes through the pulmonary circulation: it is indicated in patients with suspected or established cardiovascular disease to opacify the cardiac chambers and improve visualisation of the endocardial margins of the left ventricle5.
Doppler examination of microvasculature
SonoVue® improves diagnostic accuracy in detecting or excluding changes in cerebral arteries, extracranial carotid arteries, and peripheral arteries by improving Doppler signal-to-noise ratio.
SonoVue® improves Doppler flow image quality and duration of clinically useful signal enhancement in portal vein assessment5.
SonoVue® improves visualization of the vasculature of liver and breast lesions during echo-Doppler examination, thus allowing more specific lesion characterisation5.
Focal liver lesions
SonoVue® in the characterisation of focal liver lesions:
- very good results in terms of efficacy and comparable to CT and MRI6,7,8,9,10
Ultrasound of urinary excretory tract
SonoVue® is indicated for use in ultrasound of the urinary excretory tract in paediatric patients, from birth to 18 years of age, to detect the presence of vesicoureteral reflux. literature reference5
SonoVue® in endocardial border definition
- baseline echocardiography significantly underestimates left ventricular volumes compared to cine ventriculography and cardiac MRI11
- contrast echocardiography is particularly useful when a reproducible and accurate measurement of left ventricular function is required12
Contact us
Please fill out the form below and we will get back to you.
DISCLAIMER
For any type of product, whether drug or device, mentioned on this website, physicians should carefully review the accompanying documentation, instructions or user manual before administering it to the patient to ensure proper use. Data on the characteristics of Bracco Imaging's main products are available online.
SonoVue® should not be administered to patients with known hypersensitivity to sulphur hexafluoride or any of the excipients. The use of SonoVue® is contraindicated in patients with recent onset acute coronary syndrome or clinically unstable cardiac ischaemia, including: ongoing or evolving myocardial infarction; episodes of typical angina at rest in the 7 days preceding the examination; significant worsening of cardiac symptoms in the 7 days preceding the examination; recent coronary artery surgery or other factors suggestive of clinical instability (e.g., recent changes in ECG tracing or changes in clinical or laboratory data); acute heart failure, class III/IV heart failure, or severe arrhythmias. SonoVue® is also contraindicated in patients with known left-right shunts, severe pulmonary hypertension (pulmonary artery pressure > 90 mmHg), uncontrolled systemic hypertension, and adult respiratory distress syndrome. The safety and efficacy of SonoVue® have not been studied in pregnancy and lactation, therefore SonoVue® should not be administered during these periods.